Abstract
Introduction Aggressive natural killer cell leukemia (ANKL) is a rare leukemic form of mature NK cell neoplasm that is closely associated with the Epstein-Barr virus (EBV). Patients with ANKL show a fulminant clinical course and poor prognosis with a median overall survival (OS) of a few months after diagnosis. L-asparaginase (L-asp)-containing chemotherapies and allogeneic hematopoietic stem cell transplantation (HSCT) are considered as effective therapeutic strategies. However, owing to the rarity of this disease, the characteristics and outcomes of ANKL remain unclear.
Methods Data of 103 patients diagnosed with ANKL between 2002 and 2021 from 64 hospitals were retrospectively analyzed. Diagnosis was based on the World Health Organization classification and was verified by two co-authors (AF and RS). Patients registered in our previous ANKL studies were excluded.
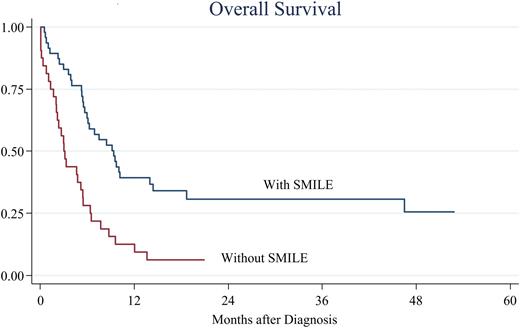
Results Patient's median age was 50 years (range, 17-90 years), and males accounted for 58%. Twelve patients (12%) had a medical history of chronic active EBV infection (9%), severe mosquito bite allergy (5%), and chronic lymphoproliferative disorder of NK cells (1%). B symptoms were commonly observed at the time of diagnosis (89%). Performance status (PS) was poor (2-4) in more than half of patients (53%). The median percentage of ANKL cells was 20.0% (range, 0-93.4) in bone marrow (BM) and 10.3% (range, 0-89) in peripheral blood (PB). ANKL cells express CD2 (95%), CD7 (53%), CD16 (41%), CD56 (95%), and EBER (86%). Fifty-two patients analyzed (58%) had chromosomal abnormalities by G-banding method, including chromosome 17 abnormalities in 19, deletion of 6q in 11, and isochromosome 7 in 5. Blood tests at diagnosis typically showed pancytopenia with a median white blood cell count of 2,900/μl, hemoglobin level of 10.3g/dl, and platelets 4.3×104/μl. High serum lactate dehydrogenase and soluble interleukin-2 receptor level were also observed (median, 882U/l and 8,475U/dl, respectively). The most common extra-nodal site of involvement was the BM (100%), followed by the spleen (84%), liver (70%), and PB (59%). Median OS of the present cohort was 5.3 months, and 1-year OS was 25.7%. Prognosis was significantly better in patients who underwent HSCT than in those who did not undergo HSCT (P < 0.001). The median OS and 1-year OS were 12.0 months and 52.3% in the former group and 1.7 months and 3.8% in the latter. Eighty-three patients received multi-agents-containing chemotherapy, while the others received single agent or did not receive any anti-lymphoma chemotherapy owing to poor general condition. The most common first-line chemotherapy was the SMILE regimen (39%), followed by anthracycline-based regimens, mainly CHOP (24%), DeVIC (15%) and other L-asp-containing regimens (8%). The response rate was highest in patients treated with SMILE (66%), followed by those who received other L-asp-containing regimens (57%), DeVIC (36%) and anthracycline-based regimens (32%). OS was significantly better in patients who received SMILE than in those who did not receive SMILE (1-year OS 39.3% vs. 12.1%, P < 0.001, Figure). Furthermore, the HSCT rate was significantly higher in patients who received SMILE (68% vs. 26%, P < 0.001). Multivariate analysis using the Cox proportional hazards model showed good PS (0-1) (hazard ratio [HR] 0.43, 95% confidence interval [CI] 0.23-0.91, P = 0.03), administration of SMILE (HR 0.43, 95% CI 0.23-0.80, P = 0.009), and HSCT (HR 0.26, 95% CI 0.12-0.58, P = 0.001) were independent factors associated with favorable OS.
Conclusion The present study showed better OS as compared with our previous study. The SMILE chemotherapy and HSCT were suggested to be key components of the therapeutic strategy for ANKL. Further studies are warranted to validate these findings.
Disclosures
Fujimoto:Chugai pharmaceutical: Honoraria; Meiji Seika Pharma: Honoraria; Sanofi: Honoraria. Maeda:Bristol Myers Squibb: Honoraria; Chugai: Honoraria; Janssen: Honoraria; Nippon Shinyaku: Honoraria; Novartis: Honoraria; Ono: Honoraria; Sanofi: Honoraria. Fukuhara:HUYA: Consultancy; Novartis: Consultancy, Honoraria; Bayer: Research Funding; Chugai pharma: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Genmab: Research Funding; Incyte: Research Funding; Dainippon Sumitomo: Honoraria; Eisai: Honoraria; Janssen: Honoraria; Kyowa kirin: Honoraria; Nippon Shinyaku: Honoraria; Ono pharma: Honoraria; Celgene: Honoraria, Research Funding; Sanofi: Honoraria; Symbio: Honoraria; Takeda: Honoraria; Eli Lilly: Consultancy; AstraZeneca: Consultancy, Honoraria; Abbvie: Consultancy. Miyazaki:Kyowa Kirin: Honoraria, Research Funding; Zenyaku Kogyo: Research Funding; AstraZeneca: Honoraria, Research Funding; Chugai Pharmaceutical: Honoraria, Research Funding; Eisai: Honoraria; Celgene: Honoraria; Nippon-Shinyaku: Honoraria; Janssen Pharmaceutical: Honoraria; Symbio: Honoraria; Bristol-Myers Squibb: Honoraria; Meiji Seika: Honoraria; AbbVie: Honoraria; Novartis Pharma: Honoraria; Takeda Pharmaceutical: Honoraria. Yamaguchi:Janssen: Honoraria; SymBio Pharmaceuticals: Honoraria; Otsuka Pharmaceuticals: Honoraria, Research Funding; AstraZeneca: Research Funding; Eisai: Research Funding; AbbVie: Honoraria; Meiji-Seika: Honoraria; Takeda Pharmaceutical: Honoraria, Research Funding; Kyowa Kirin: Honoraria, Research Funding; Nippon Shinyaku: Honoraria, Research Funding; Bristol Meyers Squibb: Honoraria; MSD: Honoraria; Ono Pharmaceutical: Honoraria; Celgene: Honoraria; Daiichi Sankyo: Research Funding; Sumitomo Dainippon: Research Funding; Genmab: Honoraria, Research Funding; Chugai Pharma: Honoraria, Research Funding. Ishida:Chugai Pharmaceuticals: Research Funding; CSL Behring: Research Funding, Speakers Bureau; Janssen: Honoraria; Pfizer: Honoraria, Speakers Bureau; Daiichi-Sankyo: Research Funding; Kyowa Kirin: Research Funding; Astra Zeneca: Speakers Bureau. Suzuki:Kyowa-Kirin: Honoraria, Research Funding; Chugai: Honoraria, Research Funding; Taiho: Research Funding; Ohtsuka: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Shionogi: Research Funding; Eisai: Honoraria, Research Funding; Bristol Meyer Squib: Honoraria; MSD: Honoraria; Celgene: Honoraria; Jansen: Honoraria; Abbvie: Honoraria; Meiji Seika: Honoraria; Sumitomo Dainippon: Honoraria; Novartis: Honoraria; AstraZeneca: Honoraria; Nippon Shinyaku: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal